Not all TAVR valves are made alike.
Your valve choice matters.
If you have severe aortic stenosis and are experiencing symptoms, you now have even more choices when it comes to which transcatheter aortic valve replacement (TAVR) valve you receive. Understanding which TAVR valve you are receiving is very important, as not all TAVR valves are created equal.
By choosing one of the Edwards SAPIEN transcatheter heart valves, you are getting one of the most widely used TAVR valves around the world, having been implanted in over 763,000 patients around the world.*
Edwards’ newest valve, the SAPIEN 3 Ultra RESILIA valve is a bovine (cow) heart tissue valve that has been treated with a special Edwards technology to block calcium build-up on the valve, potentially allowing the valve to last longer.†1
Both the SAPIEN 3 Ultra RESILIA valve and the leading surgical valves use RESILIA tissue, known to block calcium build-up on the tissue, which could potentially allow the valve to last longer.†1 Before today, the RESILIA tissue was only approved to be used on surgical heart valves.†1
The SAPIEN 3 Ultra RESILIA valve is the only TAVR valve with RESILIA tissue†1
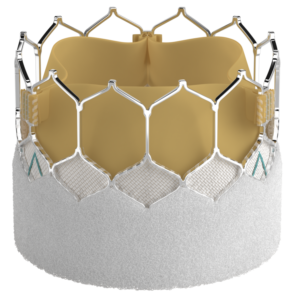
The SAPIEN 3 Ultra RESILIA valve is the latest valve approved in the SAPIEN 3 TAVR family of valves
*Currently, does not include the SAPIEN 3 Ultra RESILIA valve.
**The PARTNER 3 Trial, SAPIEN 3 TAVR proven superior to surgery on the primary endpoint of all-cause death, all stroke, and re-hospitalization (valve-related or procedure-related and including heart failure) at one year, and multiple pre-specified secondary endpoints in low risk patients.
PARTNER 3 Trial 5-Year Results in low-risk patients – Low rates of cardiovascular mortality through five years (5.5% SAPIEN 3 TAVR to 5.1% SAVR). Low rates of all-cause mortality through five years (10.0% SAPIEN 3 TAVR vs. 8.2% with SAVR). Low rates of disabling stroke through five years (2.9% SAPIEN 3 TAVR to 2.7% SAVR). Low rates of stroke through five years (5.85% SAPIEN 3 TAVR vs. 6.4% SAVR). Lower rates of rehospitalization with SAPIEN 3 TAVR through five years (13.7% vs. 17.4%).
† RESILIA tissue has not been studied for long-term results in patients.
‡ Propensity matched analysis of intermediate risk patients using VARC3 definitions of SVD and SVD related BVF at 5 years.
The type of TAVR valve you receive can impact your heart and your future.
SAPIEN 3 TAVR valves come in four unique sizes to provide a better fit for each individual patient.
Serious risks associated with the TAVR procedure, similar to those of open heart surgery, include death, stroke, serious damage to the arteries, or serious bleeding.
***The PARTNER 3 Trial, SAPIEN 3 TAVR proven superior to surgery on the primary endpoint of all-cause death, all stroke, and re-hospitalization (valve-related or procedure-related and including heart failure) at one year, and multiple pre-specified secondary endpoints in low risk patients.
PARTNER 3 Trial 5-Year Results in low-risk patients – Low rates of cardiovascular mortality through five years (5.5% SAPIEN 3 TAVR to 5.1% SAVR). Low rates of all-cause mortality through five years (10.0% SAPIEN 3 TAVR vs. 8.2% with SAVR). Low rates of disabling stroke through five years (2.9% SAPIEN 3 TAVR to 2.7% SAVR). Low rates of stroke through five years (5.85% SAPIEN 3 TAVR vs. 6.4% SAVR). Lower rates of rehospitalization with SAPIEN 3 TAVR through five years (13.7% vs. 17.4%).
Understand why your first transcatheter valve choice matters. Know the different types of valves available to you. Don’t settle for just any TAVR valve. Make sure you understand your options.
Edwards continues to build upon its best-in-class design and technology for each of its successive valves. New research confirms the short and long-term effectiveness of TAVR by Edwards, as compared to open heart surgery in low-risk patients. TAVR was proven superior at one year—and equally as effective at 5 years.**
The Edwards SAPIEN 3 Ultra RESILIA valve builds upon the best-in-class and proven SAPIEN valve platform, while also using the tissue from the leading Edwards surgical valves.
Edwards’ commitment to technology continues with the SAPIEN 3 Ultra RESILIA valve. Built using the proven SAPIEN 3 valve, the SAPIEN 3 Ultra RESILIA transcatheter heart valve is made with RESILIA tissue (previously only used on Edwards surgical valves) and designed to reduce calcium build up on the valve tissue, potentially allowing the valve to last longer.*¹
Built upon the proven SAPIEN platform, the Edwards SAPIEN 3 Ultra transcatheter heart valve was designed with patient’s future needs in mind.
Edwards’ commitment to best-in-class technology continues with the SAPIEN 3 Ultra valve. Built upon the proven SAPIEN platform, the SAPIEN 3 Ultra transcatheter heart valve was designed with patients’ future needs in mind. Its low frame height and large cell design easily facilitates future coronary access if a patient requires a procedure post-TAVR. It continues to provide alternative options for patients other than open heart surgery.
Considered best in class because of its better clinical outcomes, the Edwards SAPIEN 3 transcatheter valve became available to more patients, including those who were considered low risk for open heart surgery.¶
The Edwards SAPIEN 3 continued to advance heart valve technology, providing additional access routes and more valve sizes to meet patients’ needs. The Edwards SAPIEN 3 TAVR continued to show success in clinical data by achieving 1% death and stroke rate for those patients who are considered low-risk, which provided patients with better clinical outcomes than open heart surgery.1
Building on the success and innovation of the Edwards SAPIEN valve, the updated design of the Edwards SAPIEN XT allowed for TAVR to be used in more patients.
The Edwards SAPIEN XT valve was FDA approved to treat severe aortic stenosis patients who were experiencing symptoms and considered to be high-risk or inoperable for open heart surgery.
The Edwards SAPIEN transcatheter heart valve was the first TAVR valve of its kind to get FDA approval in the United States.
The Edwards SAPIEN 3 TAVR valve gave patients who were too sick to undergo open heart surgery an alternative treatment for severe aortic stenosis. As Edward’s first transcatheter heart valve, and the first of its kind approved in the United States, the Edwards SAPIEN 3 TAVR valve represented a major achievement in the treatment of severe aortic stenosis.
Learn about the benefits of Edwards valves and what that means for getting back to the life you love.
References
1. Flameng et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015;149:340–5.
2. Pibarot 2020 (S-13919) describes 5-yr PII S3i trial outcomes similar between S3 and SAVR, based on VARC3 definition.
*Currently, does not include the SAPIEN 3 Ultra RESILIA valve.
**The PARTNER 3 Trial, SAPIEN 3 TAVR proven superior to surgery on the primary endpoint of all-cause death, all stroke, and re-hospitalization (valve-related or procedure-related and including heart failure) at one year, and multiple pre-specified secondary endpoints in low risk patients.
PARTNER 3 Trial 5-Year Results in low-risk patients – Low rates of cardiovascular mortality through five years (5.5% SAPIEN 3 TAVR to 5.1% SAVR). Low rates of all-cause mortality through five years (10.0% SAPIEN 3 TAVR vs. 8.2% with SAVR). Low rates of disabling stroke through five years (2.9% SAPIEN 3 TAVR to 2.7% SAVR). Low rates of stroke through five years (5.85% SAPIEN 3 TAVR vs. 6.4% SAVR). Lower rates of rehospitalization with SAPIEN 3 TAVR through five years (13.7% vs. 17.4%).
†RESILIA tissue has not been studied for long-term results in patients.
§Indication for aortic THV-in-THV with demonstrated future coronary access (68/68 patients).
¶The PARTNER 3 Trial, SAPIEN 3 TAVR proven superior to surgery on the primary endpoint of all-cause death, all stroke, and re-hospitalization (valve-related or procedure-related and including heart failure) at one year, and multiple pre-specified secondary endpoints in low risk patients.
Indications:
The Edwards SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve system is indicated for relief of aortic stenosis in patients with symptomatic heart disease due to severe native calcific aortic stenosis who are judged by a Heart Team, including a cardiac surgeon, to be appropriate for the transcatheter heart valve replacement therapy.
The Edwards SAPIEN 3, SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve system is indicated for patients with symptomatic heart disease due to failing (stenosed, insufficient, or combined) of a surgical or transcatheter bioprosthetic aortic valve, a surgical bioprosthetic mitral valve, or a native mitral valve with an annuloplasty ring who are judged by a Heart Team, including a cardiac surgeon, to be at high or greater risk for open surgical therapy (i.e., predicted risk of surgical mortality ≥ 8% at 30 days, based on the Society of Thoracic Surgeons (STS) risk score and other clinical co-morbidities unmeasured by the STS risk calculator).
Contraindications (Who should not use):
The Edwards SAPIEN 3, Edwards SAPIEN 3 Ultra and SAPIEN 3 Ultra RESILIA Transcatheter Heart Valve System should not be used in patients who:
Warnings:
Precautions:
The long-term durability of the Edwards SAPIEN 3 Ultra, SAPIEN 3 Ultra RESILIA and SAPIEN 3 transcatheter heart valves are not known at this time. Regular medical follow-up is recommended to evaluate how well a patient’s heart valve is performing. Limited clinical data are available for transcatheter aortic valve replacement in patients who are born with an aortic heart valve that has only two leaflets and who are determined to be at low risk for open heart surgery. A patient’s anatomical characteristics should be considered by their physicians when using the valve in this patient population. In addition, patient age should be considered as long-term durability of the valve has not been established. Patients who need a dental procedure should talk to their doctor about risk of infection and needing antibiotics. Patients should be treated post-procedure for heart infection as a precaution.
The safety and effectiveness of the transcatheter heart valves are also not known for patients who have:
Potential risks associated with the procedure include:
Additional potential risks specifically associated with the use of the heart valves include:
CAUTION: Federal (United States) law restricts these devices to sale by or on the order of a physician.




